Development and authorisation of vaccines
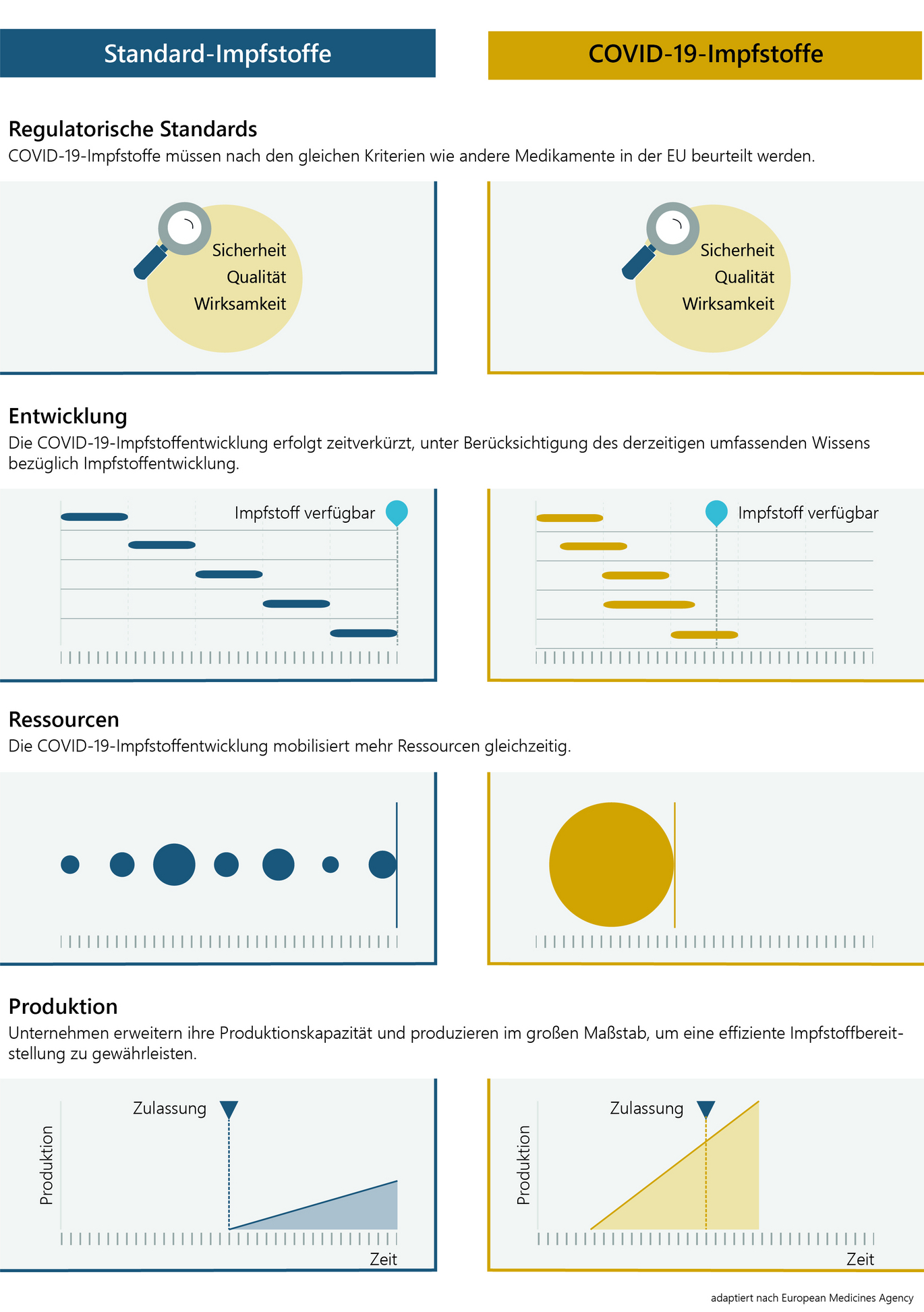
Like all medicinal products, vaccines are developed, tested and authorised in accordance with current official guidelines and legal requirements, taking into account scientific guidelines. The aim is to provide a high-quality, effective and, above all, safe vaccine.
Development of vaccines
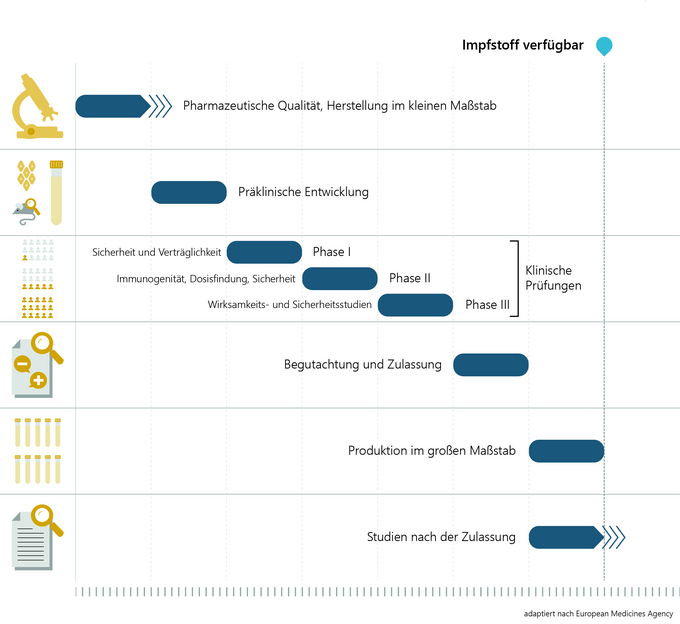
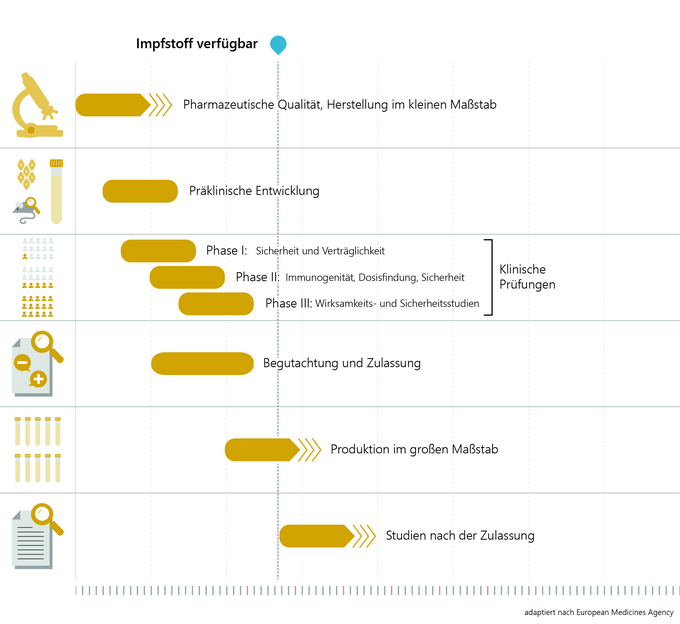
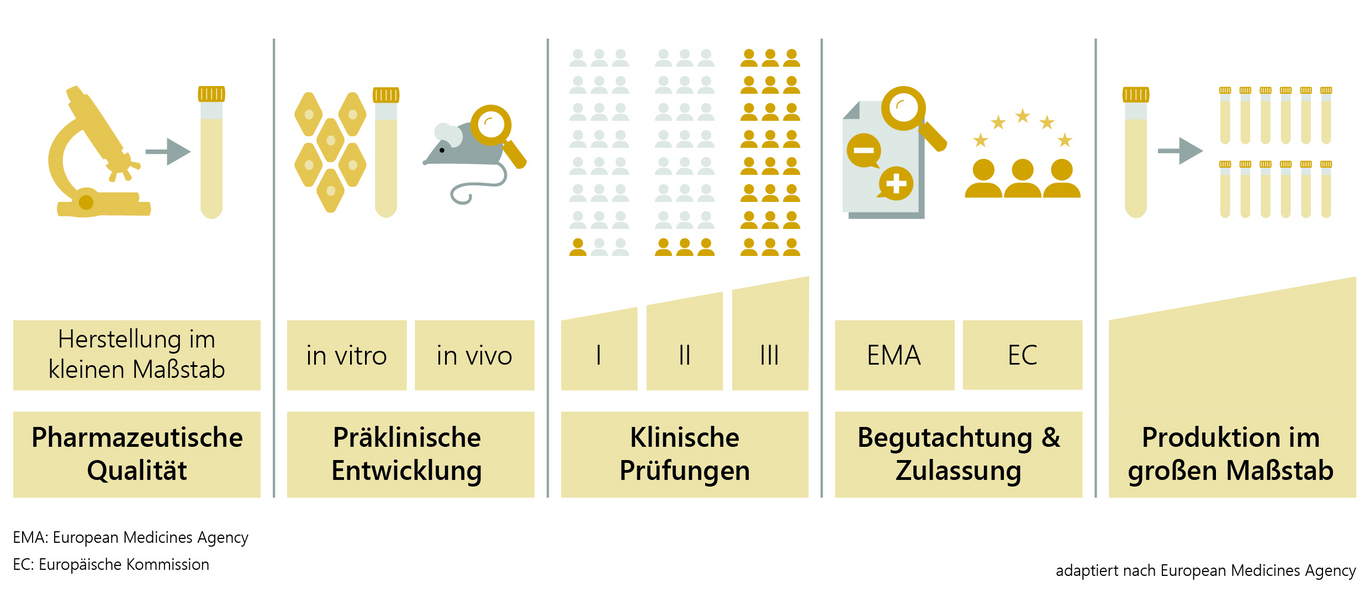
The development and research of a vaccine candidate in the laboratory takes around two to five years. First, the pathogen is analysed and tested to see which components of the virus the human immune system reacts to and can build up protection (antibodies). This is followed by the development of the vaccine and small-scale production for further vaccine testing on animals and humans.
In preclinical vaccine development, the immunogenicity (ability of the antigen to trigger immunisation), efficacy and safety of the vaccine are tested in cell cultures (in vitro, e.g. with human immune cells) and in animal experiments (in vivo). Only after extensive testing and proof that the vaccine can be produced in good quality and in accordance with all the high requirements is the vaccine tested in clinical trials on volunteers who have been informed of all possible risks. This also takes around two to five years.
Clinical trials
The competent national authorities and ethics committees ensure that all studies are conducted in a scientifically sound and ethically correct manner. Clinical trials prior to vaccine authorisation can be divided into three phases.
Phase I: As a rule, between 20 and 100 healthy volunteers are involved in order to test whether the vaccine triggers the expected reactions (immunogenicity) using laboratory tests. However, safety and tolerability are primarily tested here.
Phase II: Studies on several hundred volunteers provide information on the best possible vaccine dosage for optimum protection, the side-effect profile and the number of vaccinations required for the best possible vaccination schedule.
Phase III: In the final phase, the vaccine is tested on several thousand (in the case of COVID-19 vaccines on several 10,000) volunteers in the target population. It shows how effectively the vaccine protects against the disease compared to a control group (e.g. placebo) and what side effects may occur and with what frequency.
An official authorisation procedure is generally a prerequisite for a vaccine to be placed on the market. Authorisation means: testing a vaccine for quality, efficacy and safety on the basis of the data submitted by the applicant. In the EU, the authorisation procedure for COVID-19 vaccines is coordinated by the European Medicines Agency (EMA). The national medicines authorities are involved in the technical assessment.
The applicant submits authorisation documents for official review in accordance with clearly defined guidelines. These include regulatory information, manufacturing data, preclinical and clinical data, current specialist literature and information on the planned long-term monitoring following authorisation. In addition to the vaccine antigen, the "actual active ingredient", all other ingredients of a vaccine are also assessed. The European Pharmacopoeia, which has the character of legislation, defines these ingredients, including their permitted limits.
A vaccine is authorised if the risk-benefit ratio is positive, meaning that the benefits outweigh any risks. The "standard" authorisation procedure itself can take up to two years.
If the vaccine fulfils all scientific and regulatory requirements and its benefits outweigh its risks, the EMA's panel of experts (consisting of representatives from the EU member states, Norway and Iceland) issues a recommendation for approval of the vaccine to the European Commission following a successful approval procedure. This applies to all EU countries; national authorisation is no longer required.
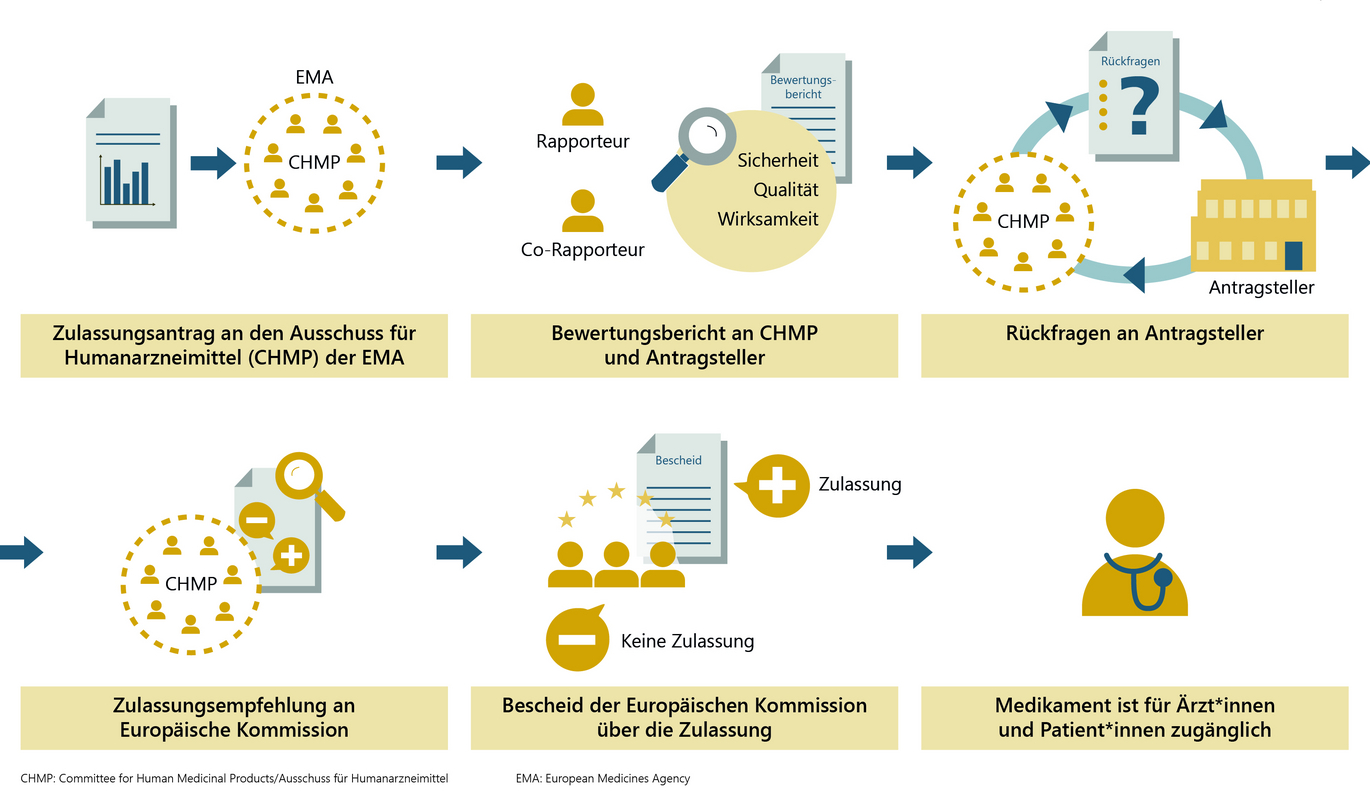
In Austria, a batch release by an officially appointed medicines control laboratory (OMCL - Offical Medicines Control Laboratory of AGES) is also required. A batch is a quantity of a vaccine produced in the course of a standardised manufacturing process. Any medicinal product control laboratory in the EU or the European Economic Area (EEA) and in Switzerland can carry out batch release for vaccines if it fulfils the legal requirements. The release certificates must then be recognised throughout the EU/EEA and Switzerland. Manufacturers can decide for themselves to which medicinal product control laboratory they submit their vaccine for batch release.
COVID-19 vaccines
The development and authorisation process for COVID-19 vaccines is being accelerated due to the serious consequences of this pandemic. Extensive existing knowledge about coronaviruses and vaccine development is being utilised.
In order to accelerate development, companies and research institutions are deploying significantly more personnel and financial resources in a shorter period of time than in conventional development processes. Where this is possible while complying with strict safety requirements, study phases are also carried out in parallel. In addition, manufacturers are expanding their production facilities at a much earlier stage of vaccine development than usual so that large quantities of vaccine are available quickly after authorisation.
In addition, the European Medicines Agency (EMA) offers accelerated scientific advice for vaccine developers in order to drive development forward in a targeted and focussed manner.
mRNA vaccines
Some vaccines against COVID-19 are being developed using new technologies that are expected to increase the quantities and speed of production compared to other methods of vaccine production. One of these new approaches is based on messenger RNA (mRNA). A small part of the "blueprint" of the virus, which consists of RNA (ribonucleic acid), is produced in the laboratory and added to the vaccine. With this blueprint, cells of the human body can produce a part of the virus - a so-called surface protein - which, unlike the virus, cannot trigger an infection and therefore no resulting Covid-19 disease. The immune system recognises this surface protein as foreign and produces antibodies. If a vaccinated person becomes infected with COVID-19, the immune system is already prepared and can fight the pathogen much better. The mRNA in the vaccine is broken down again by the body within a short period of time, which also ends the production of the surface protein. The mRNA cannot have any influence on the human genome, which consists of DNA. The two cannot bind to each other and, moreover, the mRNA only reaches the cell area outside the cell nucleus where the DNA is located.
Accelerated authorisation procedure
The sooner a safe and effective COVID-19 vaccine is available, the sooner more serious effects of the current pandemic can be prevented. However, the benefits of a vaccine to protect against COVID-19 must far outweigh the potential risks and side effects. The authorisation procedure ensures this - even in an accelerated process - on the basis of independent scientific assessments by the Medicines Agency. In addition, close monitoring is also carried out after vaccine authorisation.
In the case of COVID-19 vaccines, there are no compromises or differences to the "conventional" authorisation process in terms of the quality, type and scope of the official assessment. However, this will be accelerated with the following three measures in order to make urgently needed vaccines available.
1) Accelerated authorisation procedure
The maximum time frame for a standard authorisation procedure is 210 days, during which the authorisation authority actively works on the assessment of the submitted data. The maximum timeframe for the accelerated procedure is 150 days. The assessment is carried out in the same steps and with the same quality, but the authorities' teams use significantly more resources in a shorter period of time.
2) Rolling Review
In the case of promising vaccine candidates, the authorisation authorities can begin to review existing data packages in parallel with the ongoing development. The subsequent "actual authorisation procedure" can then be completed in a shorter time, as large parts of the data have already been reviewed in detail.
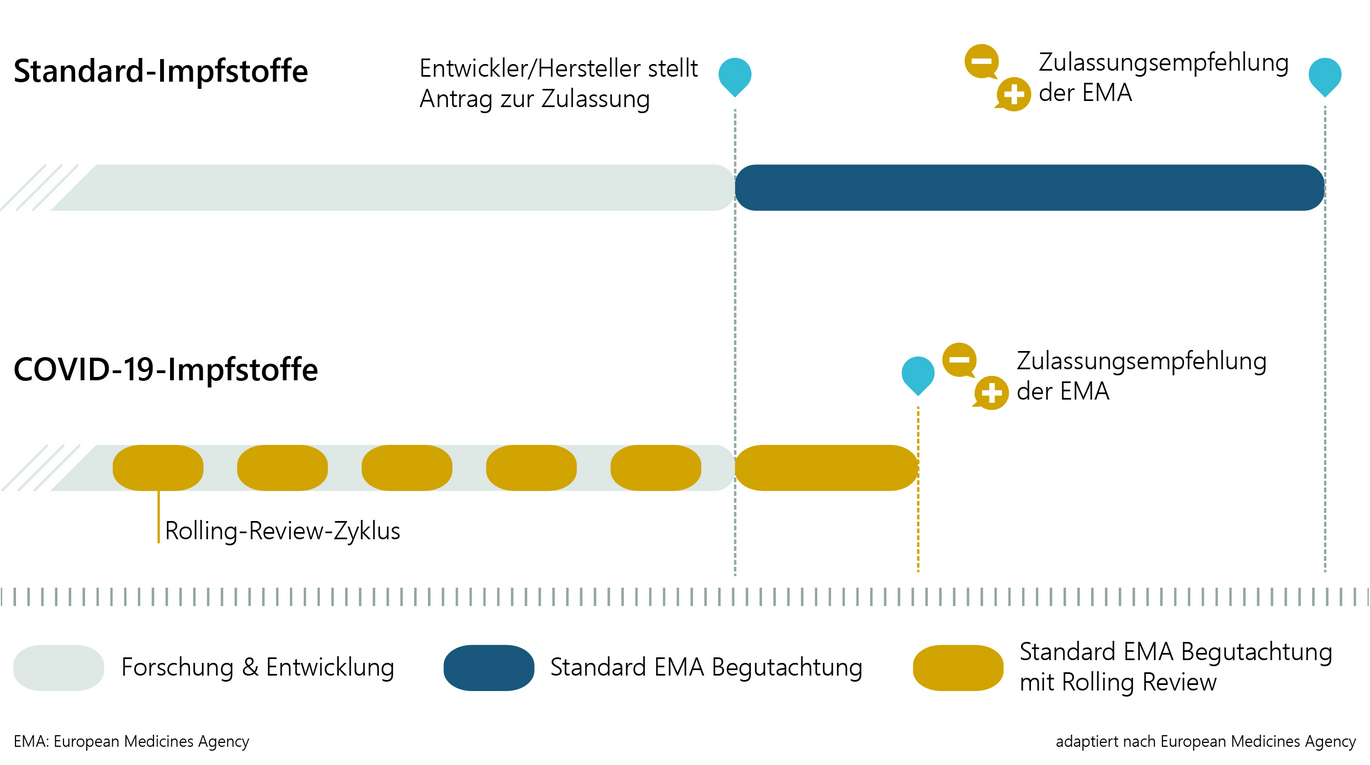
3) Conditional Marketing Authorisation
This special form of "ordinary authorisation" can only be chosen for medicinal products for which there is an urgent need due to an emergency situation that cannot currently be met - this is the case for the current Covid-19 pandemic. The applicant must first submit sufficient data to demonstrate the quality, safety and efficacy of the vaccine and enable a risk-benefit assessment. Certain data and information, which must be precisely defined at the time of authorisation, must be submitted for review once authorisation has been granted. This is subject to strict conditions and specifications as to when the outstanding information must be submitted to the authorities.
The conditional authorisation is valid for one year and can be extended if necessary. As soon as all conditions and requirements have been met, the conditional authorisation can be converted into a "standard" authorisation.
Regardless of which options are used to speed up the procedure, the risk-benefit assessment by the EMA's expert committee decides whether or not authorisation can be recommended. If the assessment is positive and therefore a recommendation for authorisation is made, the European Commission issues the marketing authorisation decision.
Vaccine safety after authorisation
Vaccines are continuously monitored not only before and during authorisation, but also while they are on the market. Pharmacovigilance refers to a variety of methods and activities that are intended, among other things, to enable the detection, assessment, understanding and prevention of side effects.
Part of pharmacovigilance is the reporting obligation of healthcare professionals in connection with the use of vaccines. In the case of medicinal products for human use, it applies to suspected adverse reactions and also to the absence of the expected efficacy. However, not only healthcare professionals, but also patients and their relatives can report suspected adverse reactions. Reports must be submitted electronically or in writing to the Federal Office for Safety in Health Care (BASG).
All reports of suspected adverse reactions are collected throughout the EU. Analysing all this data makes it possible to identify a possible new risk at national and European level (signal detection), to examine it closely and thus contribute to greater drug safety for all patients. If a signal is detected, it is assessed in the European context by the EMA's Pharmacovigilance Risk Assessment Committee (PRAC). Depending on the outcome of the assessment, measures such as the inclusion of new warnings in the product information leaflet or, in extreme cases, even the cancellation of a vaccine's marketing authorisation are taken.
Vaccine information from the BASG
Further information on medicinal products, vaccines and medical devices can be found on the website of the Federal Office for Safety in Health Care (BASG).
Instructions for use and technical information on the authorised COVID-19 vaccines can also be found here at the BASG.
Last updated: 08.02.2024
automatically translated