Human Medicine
When it comes to human health, safety has top priority. With our highly qualified experts and state-of-the-art equipment, we can offer precisely this safety in human medicine at the highest level. For example, for pharmacies in the microbiological testing of pharmaceuticals. Furthermore, in the field of hygiene consulting in hotels, homes, hospitals and other health care facilities with regard to prevention and occupational safety.
Numerous operators of hospitals, health centers as well as pharmaceutical companies and pharmacies already rely on the first-class expertise of AGES. As a state-accredited* testing and conformity assessment body, we guarantee that all our services in the field of human medicine meet the required technical, professional and quality assurance requirements. In addition, you benefit from:
- first-class consulting in solving individual problems
- very short analysis times
- rapid reporting
- well-founded elaboration of recommendations and statements
Microbiological tests
Manufacturers of medicinal products (AM) and medical devices (MP) must ensure before placing their products on the market that patients are not exposed to any risk due to insufficient safety, quality or efficacy. An appropriate quality assurance system should ensure that AMs are not sold or dispensed until a competent person has certified that each manufacturing batch has been manufactured and tested in accordance with the requirements specified in the marketing authorization and all other regulations relevant to the manufacture, testing and release of AMs. Pharmaceutical companies, healthcare facilities or pharmacies thus require microbiological testing in accordance with pharmacopoeias and applicable medical/pharmaceutical standards or guidelines.
Microbiological testing of non-sterile drugs and medical devices
Our offer
More than 10,000 non-sterile AM are tested annually in the laboratories of the General Microbiology and Hygiene Department in Graz. The department is certified according to GMP and c-GMP and holds an accreditation* as a testing laboratory according to EN ISO 17025. Thus, our analytical procedures are subject to strict national laws (AMG), regulations (AMBO) and standards and comply with the highest international standards (e.g. CFR of the FDA = US Food and Drug Administration). In the pharmaceutical field, our department offers a wide range of tests that are primarily based on the requirements of pharmacopoeias (Eu. Ph., European Pharmacopoeia, USP, American AB, German AB, British AB etc.). In addition to our comprehensive testing services, our customers, which include many well-known drug manufacturers, benefit from our extensive expertise and consulting competence. The following areas can be covered in the testing of non-sterile products by AGES:
- Testing of microbial contamination (quantitative and qualitative) and detection of specific germs
- Germ identification by means of microbiological, biochemical and molecular biological methods
- Growth control and sterile testing of culture media
- Provision of nutrient media
- Testing for sufficient antimicrobial preservation
- Detection and quantification of endotoxins
- Testing of sterilization processes (bioindicators)
- Testing of disinfectants (challenge test)
- Testing of in-use stability with sampling
- Examination of pharmaceutical waters
- Microbiological investigations in the course of plant qualification (including environment, water)
- Environmental testing
- Development of the test method for the respective product
- Verification of the suitability of the method for the respective product (suitability test, validation)
- Preparation of standard-compliant test reports
- Consulting for the solution of individual questions and problems
Microbiological testing of sterile drugs and medical devices
Our offer
More than 10,000 sterile drugs are tested annually in the laboratories of the General Microbiology and Hygiene Department in Graz. The department is certified according to GMP and c-GMP and holds an accreditation* as a testing laboratory according to EN ISO 17025. Thus, our analytical procedures are subject to strict national laws (AMG), regulations (AMBO) and standards (e.g. for medical devices) and meet the highest international standards (e.g. CFR of the FDA = US Food and Drug Administration).
In the pharmaceutical field, the MIHY department offers a wide range of analyses, which are primarily based on the requirements of pharmacopoeias (especially Eu. Ph., European Pharmacopoeia, USP, American AB).
The analysis of sterile AM/MP requires a high degree of purity. In order to avoid cross-contamination, the tests are therefore also carried out in controlled clean rooms.
The testing itself is specifically adapted in our laboratory to the respective product and to your requirements. In addition to our comprehensive testing services, our customers, which include many well-known pharmaceutical manufacturers, benefit from our extensive expertise and consulting competence in pharmaceutical and medical products.
The following areas can be covered when testing for sterility by AGES:
- Performance of sterility testing (membrane filtration, direct loading) according to Eu. Ph. 2.6.1 and USP 71>
- Development of the test method for the respective product
- Verification of the suitability of the method for the respective product (suitability test, validation, stasis test).
- In case of detection of product contamination, germ identification by means of microbiological, biochemical and molecular biological methods
- Preparation of standard-compliant test reports
- Consulting for the solution of individual questions and problems
The range of products we examine for you includes:
- Pharmaceuticals in human and veterinary medicine, (surgical) medical devices
- Bone tissue, blood and cell fluids
- Isotope diagnostics
- Syringes, infusions and infusion bags
- Tube feeds
- Aqueous solutions (e.g. injectables), soluble powders
- Ointments, creams, oils and oily solutions (e.g. ophthalmics)
Training legionella & practice sampling
The ÖNORM B 5019 "Hygienic planning, execution, operation, monitoring and renovation of central drinking water heating systems" describes a largely safe installation as well as the safe operation of drinking water heating systems to prevent the possibility of infection by Legionella pneumophila and other species. The training is intended to increase awareness of this topic. If desired, the practical part for the technical staff focuses on the execution of correct sampling. All participants receive a certificate of participation or training certificate from the AGES Academy.
Our offer
The training takes place either on site or in AGES' own training facilities. The practical part is carried out on request. The following areas can be covered by AGES during the short training course:
- Theoretical part:
- Overview of origin, spread, impact of Legionnaires' disease (Legionella pneumonia).
- Overview of standards and regulations that must be observed or followed for the operation and maintenance of drinking water heating systems
- Inspection of drinking water heating systems
- Practical part for technical staff (on request):
- Implementation of proper sampling and sample handling (storage and transport) for the purpose of analysis.
- On-site training in the company or other formats on requestCertificate of participation or training certificate from the AGES Academy .
Water hygiene
Clean, safe water is a precious commodity. Operators of a public water supply system, a health or wellness centre, hotels and also property management companies have a great responsibility in this regard. Specifically, it is their task to fulfil the strict legal and normative requirements. Our highly qualified experts provide competent and reliable support.
Our Institute for Hydroanalysis, which specialises in water hygiene, is accredited as a testing and inspection body and is the first port of call when it comes to having the safety of water confirmed or taking appropriate measures to this end:
- You can rely on our extensive scientific expertise and practical experience in physical, chemical and microbiological water analysis.
- Benefit from professional advice and support in all matters that are legally required and necessary for perfect water quality. Customised, flexible, fast on the basis of our consistent, process-oriented quality management.
Inspection of water supply systems
The institutes in our Public Health division (Institute for Hydroanalytics Linz, Institute for Medical Microbiology and Hygiene Vienna and Institute for Medical Microbiology and Hygiene Graz) are accredited as test centres in accordance with EN ISO 17025 and as a joint inspection body for the division in accordance with EN ISO 17020. In a detailed consultation, we clarify your exact testing requirements, such as a chemical and microbiological examination or testing for harmful substances such as pesticide residues. Based on this, we carry out a thorough inspection and prepare professional test and inspection reports, including an expert opinion in accordance with food law.
What we offer
Operators of water supply systems must have the quality of water for human consumption tested in accordance with the Drinking Water Ordinance BGBl. II No. 304/2001 as amended (TWV). Our experienced AGES experts carry out the inspection in accordance with ÖNORM M 5874 on the basis of the official decision on the scope and frequency of the inspection. In addition to the functional testing of the water treatment plants, we analyse the water samples for all parameters of the TWV and the Austrian Food Code (Codex Chapter B1 Drinking Water). This includes microbiological tests (e.g. Escherichia coli, enterococci, coliform bacteria, Pseudomonas aeruginosa, legionella, etc.), the analysis of standard chemical parameters (e.g. pH value, electrical conductivity, water hardness, calcium, magnesium, sodium, potassium, chloride, nitrate, sulphate, nitrite, ammonium, iron, manganese, etc.), special and trace chemical parameters (e.g. pH value, electrical conductivity, water hardness, calcium, magnesium, sodium, potassium, chloride, nitrate, sulphate, nitrite, ammonium, iron, manganese, etc.).), special and trace chemical analyses (e.g. heavy metals, BTX, disinfection by-products, pesticide residues, PFAS, TFA, bisphenol A and other endocrine disruptors, etc.) and radioactivity (total direct dose, radon, radium isotopes, etc.). Finally, you will receive a professional test or inspection report and an expert opinion in accordance with food law.
We can offer the following services for drinking water analyses:
- Consultation to clarify the order
- Inspection of the drinking water supply systems
- Analysis of all parameters of the Drinking Water Ordinance and the Austrian Food Code
- Functional testing of water treatment plants
- Preparation of accredited test and inspection reports and food law expertises
Expertise in accordance with the Bathroom Hygiene Ordinance
Bathing water analysis of outdoor and indoor pools, hot tubs (whirlpools) and small bathing ponds
As part of the bathing water examination, we clarify your exact examination requirements in a detailed consultation, such as a chemical and microbiological examination or problems with the water treatment (e.g. filter contamination). Based on this, we carry out a thorough inspection and prepare accredited inspection reports and water hygiene reports.
Our offer
§ Section 14 of the Bathing Water Hygiene Act regulates bathing water inspections of outdoor and indoor pools, hot tubs (whirlpools) and small bathing ponds. Our experienced experts carry out inspections and take samples to determine the suitability of the water for use as bathing water. In addition to the functional testing of pools, whirlpools and small bathing ponds, we analyse the bathing water of outdoor and indoor pools as well as therapy pools in accordance with the Bathing Water Hygiene Ordinance. This includes microbiological testing (Escherichia coli, enterococci, Pseudomonas aeruginosa, legionella, salmonella), testing of standard chemical parameters (free and combined chlorine, pH value, chloride, nitrate, oxidisability, flocculant residues, trihalomethanes, etc.) and testing of individual issues (filter contamination, etc.). Finally, you will receive a professional test report and a water hygiene report.
We can offer the following areas of bathing water testing:
- Consultation to clarify the order
- Inspections of pools, whirlpools and small bathing ponds in accordance with the Bathing Water Hygiene Ordinance
- Analysis of bathing water in outdoor and indoor pools and therapy pools
- Microbiological analysis of standard chemical parameters
- Advice on solving individual questions and problems
- Preparation of accredited inspection reports and water hygiene reports
Institute for Hydroanalysis Linz
We offer state-of-the-art equipment, a highly qualified team, accreditation as a conformity assessment body by Akkreditierung Austria (EN ISO/IEC 17025, EN ISO/IEC 17020), short analysis times and rapid reporting and guarantee you test reports and expert opinions that are suitable for submission to the authorities.
Accredited area as inspection body according to EN ISO/IEC 17020
- Inspections of drinking water supply systems (ÖNORM M 5874) with food law reports
- Inspections of pools, whirlpools and small bathing ponds in accordance with the Bathing Hygiene Ordinance as amended.
Accredited testing centre in accordance with EN ISO/IEC 17025
- Drinking water in accordance with the Drinking Water Ordinance
- Bottled water (mineral and spring water, table water, soda water)
- Bathing water (outdoor and indoor pools, therapy pools, etc.) in accordance with the Bathing Water Hygiene Ordinance
- Bathing water
- Water for medical-pharmaceutical applications (dialysis water, ultrapure water according to PharmEU, steam sterilisers)
- Microbiology (analysis of faecal indicator bacteria, legionella in hot water, bathing water...)
- Process water (heating and cooling water, boiler feed water)
- Chemical speciality and trace analysis (heavy metals, PAH, disinfection by-products, pesticide residues, PFAS, TFA, bisphenol A and other endocrine disruptors, cyanotoxins, etc.)
Range of services in the non-accredited area
We are also at your disposal for technical questions (non-accreditable area) such as functional tests of water treatment plants (softening, deferrisation and disinfection plants) and advise you on the solution of individual questions and problems.
Note: The test centre has been accredited by Accreditation Austria in accordance with EN ISO/IEC 17025, the type A inspection body in accordance with EN ISO/IEC 17020 for the areas listed in the notification and published under Accreditation Austria.
Institute for Medical Microbiology and Hygiene Graz
We offer accreditation as a conformity assessment body by Accreditation Austria (EN ISO/IEC 17025, EN ISO/IEC 17020), rapid reporting and guarantee test reports and expert opinions suitable for submission to authorities.
Range of services in the accredited area as inspection body according to EN ISO/IEC 17020
- Inspections of drinking water supply systems according to ÖNORM M 5874
- Inspections of pools, whirlpools and small bathing ponds according to the Bathing Hygiene Ordinance as amended.
Range of services in the accredited area as a testing laboratory according to EN ISO/IEC 17025
- Examination of
- Drinking water according to the Drinking Water Ordinance as amended.
- Hot water according to ÖNORM B5019
- Bathing water (pool baths, whirlpools and small bathing ponds) in accordance with the Bathing Hygiene Ordinance, as amended.
- Bathing water according to ÖNORM 6230
- Mineral and spring water in accordance with the Mineral Water and Spring Water Ordinance, as amended.
- various chemical, microbiological and physical parameters in waters
A large part of the chemical analysis is carried out at the AGES Institute for Hydroanalytics in Linz.
Range of services in the non-accredited area
- Investigation of the chemical technical suitability of supply systems
Note: The testing laboratory has been accredited according to EN ISO/IEC 17025 as a testing laboratory with the identification number 0452, the inspection body of type A according to EN ISO/IEC 17020 with the identification number 0406 by Accreditation Austria for the areas listed in the notification and published at https://www.bmaw.gv.at/Services/Akkreditierung/Akkreditierung_Austria.html.
Institute for Medical Microbiology and Hygiene Vienna
We offer state-of-the-art equipment, a highly qualified team, accreditation as a conformity assessment body (ID 0260 and ID 0406) by Accreditation Austria (EN ISO/IEC 17025, EN ISO/IEC 17020), short analysis times and rapid reporting, and guarantee test reports and expert opinions suitable for submission to authorities.
Accredited area as inspection body according to EN ISO/IEC 17020
- Inspections of drinking water supply systems (ÖNORM M 5874) with expert opinions in accordance with food law on the basis of the Drinking Water Ordinance as amended and the Austrian Food Code Chapter B1
- Inspections of pool baths and whirlpools according to the Bath Hygiene Ordinance as amended
Accredited area as testing laboratory according to EN ISO/IEC 17025
- Drinking water according to the Drinking Water Ordinance (as amended)
- Mineral and table water according to Mineral Water and Spring Water Ordinance (as amended)
- Bathing water (outdoor and indoor pools, therapy pools, etc.) according to the Bathing Hygiene Ordinance
- Bathing water
- Water for medical-pharmaceutical applications (dialysis water, ultrapure water according to PharmEU, steam sterilizers, deionized water, feed water)
- Microbiology (examination of fecal indicator germs, legionella in hot water according to ÖNORM B 5019, ...)
- process water (heating and cooling water)
- various chemical, microbiological and physical parameters in waters
A large part of the chemical analysis is carried out at the AGES Institute for Hydroanalytics in Linz.
Range of services in the non-accredited area
We are also available for technical questions (non-accreditable area) such as functional tests of water treatment plants (softening, deferrization and disinfection plants) and advise you on the solution of individual questions and problems.
Information on legionella findings
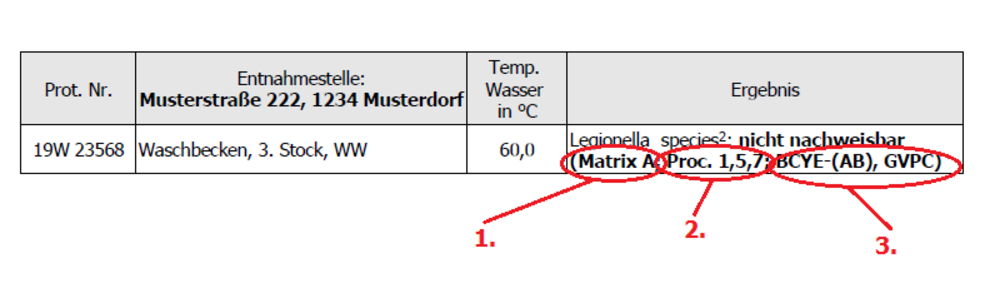
Legionella are bacteria that are widespread in nature and occur naturally in groundwater, surface water and moist soil and can thus enter drinking water in low concentrations. In drinking water heating systems, poorly thermally insulated cold water systems, poorly maintained pool baths, cooling towers and similar systems, these bacteria can multiply in biofilms (layers of slime in which microorganisms are embedded) at temperatures between 25 °C and 45 °C and stagnant water. If these bacteria are inhaled with fine droplets of water, known as aerosols, they can cause severe pneumonia, known as Legionnaires' disease. Drinking water contaminated with legionella does not pose a health risk. Legionella cannot be transmitted from person to person.
Explanation of terms:
- Matrix A: Sample material with low accompanying flora (naturally occurring microorganisms in water), for example, drinking water (hot and cold), wash water, and disinfected water such as water from pool baths, whirlpools, and whirlpool tubs, and treated pool water. Matrix B: Sample material with high accompanying flora (naturally occurring microorganisms in water), e.g. waters from cooling towers, process waters and waters from dental units.
- Proc.: = "Procedure"; indicates the procedure by which the sample is prepared and analyzed in the laboratory.
- BCYE, BCYE-AB, GVPC: indicate which culture media were used (BCYE = Legionella BCYE agar with L-cysteine, BCYE-AB = Legionella BCYE agar with supplements, GVPC = GVPC agar).
Food Microbiology
Our Food Microbiology Department at the Institute of Medical Microbiology and Hygiene Graz offers the following services:
- Detection / quantification / identification of pathogenic and hygiene-relevant microorganisms in foodstuffs
- Assessment of foodstuffs
- Consultation on questions of microbiology and hygiene (e.g. swab samples)
Sample collection hours: Monday to Thursday from 07:30 - 14:00 h, Friday from 07:30 - 12:00 h.
You can find sample slips and forms here
Contact
Leitung
Priv.-Doz. Mag. Dr. Alexander Indra
- humanmed.wien@ages.at
- +43 50 555-37111
-
Währingerstraße 25a
1090 Wien
Kontakt Wasseruntersuchungen:
DI Dr. Walter Pribil
- wasser.wien@ages.at
- +43 50555 37111
-
Währingerstraße 25a
1090 Wien
Kontakt Wasseruntersuchungen:
DI (FH) Birgit Huemer
- hydroanalytik@ages.at
- +43 50 555 - 41602
-
Wieningerstraße 8
4020 Linz
Leitung
Priv.-Doz. Dr. Burkhard Springer
- humanmed.graz@ages.at
- +43 50 555-61202
-
Beethovenstraße 6
8010 Graz
Kontakt Wasseruntersuchungen:
Dipl. Ing. Bernd Obenaus
- bernd.obenaus@ages.at
- +43 50 555-61305
-
Beethovenstraße 6
8010 Graz
Kontakt Lebensmittelmikrobiologie:
Mag. Dr. Claudia Schlagenhaufen
- lebensmittel.graz@ages.at
- +43 50 555 61310
-
Beethovenstraße 6
8010 Graz
Last updated: 23.04.2025
automatically translated